The Inner Laboratory: Cellular Metabolism and the Future of Preventive Medicine
Preventive healthcare increasingly depends on our ability to understand processes that occur at the cellular and molecular level. While laboratory assays and imaging provide snapshots of physiology, the most fundamental determinants of health reside inside cells: in mitochondria, protein quality control systems, and the molecular balance that maintains genomic integrity. These invisible processes form a hidden “laboratory within” that continuously shapes resilience, aging, and disease risk.
By connecting lifestyle, nutrition, and medical interventions to cellular mechanisms, medtech platforms can translate molecular biology into actionable preventive strategies.
Mitochondria as Energy Integrators
Mitochondria remain central to any discussion of longevity. Beyond producing ATP, they act as sensors of cellular stress, integrating signals from nutrients, oxygen, and inflammatory pathways. Dysfunction in mitochondrial respiration is implicated in neurodegeneration, cardiovascular disease, and accelerated aging [1].
Time-series metabolomics and real-time respirometry are increasingly combined with AI to model mitochondrial efficiency as a biomarker of systemic health. For instance, shifts in NAD⁺/NADH balance or accumulation of reactive oxygen species (ROS) can be quantified and predicted before functional decline is clinically evident [2].
Proteostasis and Cellular Quality Control
Cells rely on precise control of protein folding, modification, and degradation. The proteostasis network—including chaperones, the ubiquitin-proteasome system, and autophagy pathways—ensures that misfolded or damaged proteins are cleared efficiently. When this system falters, toxic aggregates accumulate, contributing to diseases such as Alzheimer’s and Parkinson’s [3].
Medtech approaches now include assays that quantify circulating proteostasis markers and AI models that link them to lifestyle and pharmacological interventions. Strength training, caloric modulation, and compounds that modulate autophagy (e.g., spermidine) have all been shown to maintain proteostasis and support healthy aging [4].
Epigenetic Regulation and Methylation
Methylation patterns in DNA and histones act as regulators of gene expression and are increasingly used as biomarkers of biological age. Unlike genetic mutations, epigenetic modifications are reversible, making them attractive targets for preventive medicine [5].
Large-scale epigenomic datasets are now integrated by machine learning to predict risk trajectories for metabolic syndrome, cancer, and neurodegenerative disorders. Interventions such as exercise, nutritional methyl donors (folate, B12), and even targeted epigenetic therapies have been shown to alter methylation age scores [6].
Telomere Dynamics
Telomeres, the protective caps at chromosome ends, shorten with each cell division, acting as a molecular clock. Excessive telomere erosion is linked to premature cellular senescence and increased risk of chronic disease [7].
AI models can combine telomere length measurements with other omic data to forecast biological aging more accurately than telomere length alone. Lifestyle interventions—including physical activity and stress reduction—have demonstrated measurable effects on telomere dynamics [8].
Nutrient and Coenzyme Networks
Cellular metabolism depends on a complex interplay of coenzymes such as NAD⁺, FAD, and coenzyme Q10. These molecules function not only in energy production but also in signaling cascades that regulate repair and inflammation [9].
Recent advances in metabolomic profiling now allow fine-grained quantification of these coenzyme states in vivo. AI-driven models can use these signatures to identify deficiencies or imbalances before they manifest clinically. This creates opportunities for personalized supplementation strategies guided by real-time data rather than generalized population norms [10].
Integrating Molecular Signals into Preventive Medtech
The challenge is not only to measure these processes but to integrate them into predictive models of health. Combining mitochondrial markers, proteostasis indicators, methylation clocks, telomere length, and coenzyme states produces a multi-dimensional profile of cellular resilience.
With AI integration, these profiles can be turned into health maps that guide preventive interventions. For example:
- A decline in NAD⁺ and mitochondrial efficiency may prioritize exercise and supplementation strategies.
- Early proteostasis dysfunction could flag the need for targeted nutritional interventions or pharmacological modulation.
- Accelerated methylation aging may lead to personalized lifestyle coaching focused on sleep and stress reduction.
By aligning these insights with wearable sensor data and clinical biomarkers, medtech platforms can provide clinicians and patients with actionable, personalized roadmaps for maintaining cellular health.
Challenges and Future Directions
Several obstacles remain before these cellular signals can be translated into routine preventive medicine:
- Standardization: Different assays for methylation, telomere length, or mitochondrial function produce heterogeneous results [11].
- Data integration: High-dimensional omics data require robust computational frameworks to avoid false correlations.
- Accessibility: Advanced assays remain costly, limiting their application outside research or specialized clinics.
- Clinical adoption: Physicians must trust these cellular metrics as actionable, not speculative, indicators.
Despite these challenges, the trajectory is clear. Medicine is moving toward a model where preventive strategies are rooted not only in population risk factors but in the molecular realities of each individual’s cells.
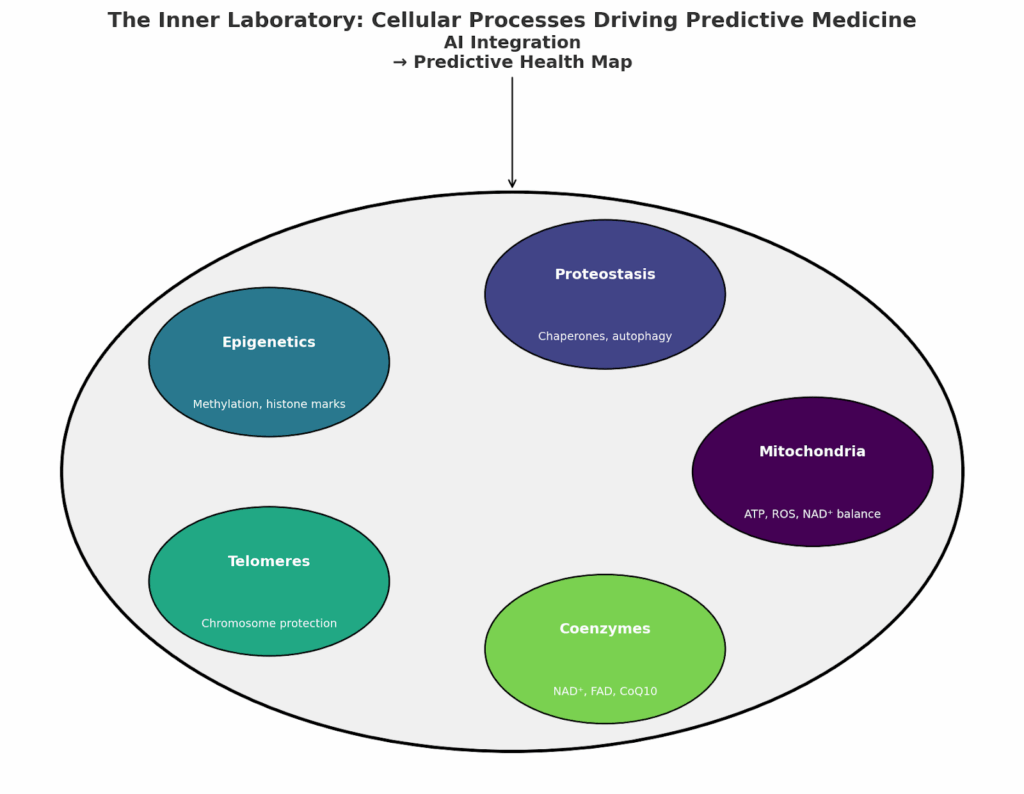
Fig. 1. Health map breakdown for predictive health analytics
Conclusion
The future of preventive medicine lies in the translation of cellular processes into measurable, actionable health signals. Mitochondrial efficiency, proteostasis, methylation, telomere dynamics, and coenzyme networks together form a hidden laboratory inside each cell. When integrated by AI into health maps, these signals can guide precise, proactive interventions.
By bridging molecular biology with medtech innovation, we are approaching a healthcare model that treats aging and disease not as inevitable outcomes but as modifiable processes visible years before symptoms appear.
References
- Sun, N., Youle, R. J., & Finkel, T. (2016). The mitochondrial basis of aging. Molecular Cell, 61(5): 654–666.
- Verdin, E. (2015). NAD⁺ in aging, metabolism, and neurodegeneration. Science, 350(6265): 1208–1213.
- Balch, W. E., et al. (2008). Adapting proteostasis for disease intervention. Science, 319(5865): 916–919.
- Madeo, F., et al. (2018). Spermidine in health and disease. Science, 359(6374): eaan2788.
- Horvath, S., & Raj, K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6): 371–384.
- Quach, A., et al. (2017). Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging, 9(2): 419–446.
- Blackburn, E. H., et al. (2015). Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature Medicine, 21(10): 1169–1173.
- Puterman, E., et al. (2018). Physical activity and telomere length in adults: a meta-analysis. Sports Medicine, 48(3): 651–670.
- Wallace, D. C. (2010). Mitochondrial DNA mutations in disease and aging. Environmental and Molecular Mutagenesis, 51(5): 440–450.
- Kennedy, B. K., & Lamming, D. W. (2016). The mechanistic target of rapamycin: the grand conductor of metabolism and aging. Cell Metabolism, 23(6): 990–1003.
- Belsky, D. W., et al. (2020). Quantification of biological aging in humans. Nature Aging, 1: 123–137.
Author
Dmitry Chebanov, Scientist, Co-Founder and Head of Health at Holiverse, Expert in Preventive Medicine and Brain Physiology.